Finder Spotlight
Israeli Innovation in Clinical Trial Operations
Written by: Yael Pomerantz, PhD, Health Tech Sector Lead, Startup Nation Central | Einat Ben Ari, Senior Director of Data & Insights, Startup Nation Central | Assaf Mischari, Managing Partner, Team8 | Dikla Shpangental, VP Global Account Management & General Manager, IQVIA Israel



Overview
Israel has emerged as a global leader in clinical trial innovation, leveraging its advanced healthcare infrastructure, technological expertise, and diverse population. The clinical trial ecosystem has grown significantly, with over 50 companies, more than double the amount in 2016 and triple the amount in 2014. Around 50% of these are early-stage companies, 29% are in the early growth stage, and 20% are mature companies, reflecting both innovation and established industry leadership.
There are five key segments the Israeli clinical trial operations tech landscape:
- Novel Trial Designs and Patient Stratification
- Patient Recruitment and Retention
- Safety and Pharmacovigilance
- Data Management and Analytics
- Logistics and Regulatory Affairs
By combining interdisciplinary innovation with robust healthcare capabilities, Israel continues to set the standard for quality and efficiency in the evolving landscape of clinical research.
Global Trends in Clinical Trial Operations Technology
The clinical trial landscape is evolving rapidly, driven by advancements in technology and a shift toward more personalized, efficient methodologies. However, these advancements are also a response to significant challenges that have long plagued the industry, including declining trial productivity, rising costs, and increasing complexity.
The Clinical Development Productivity Index – a key measure combining trial success rates, complexity, and duration – highlighted a worrying trend of declining productivity until 2021. Lengthier trials and lower success rates resulted in extended development timelines and higher R&D costs. However, innovation has begun to reverse this trend, with the index starting to rebound in 2022 the momentum continuing in 2023.
Clinical development productivity continued to increase in 2023 driven by an increase in success rates
Clinical Development Productivity Index and elements of productivity indexed to 2010 values
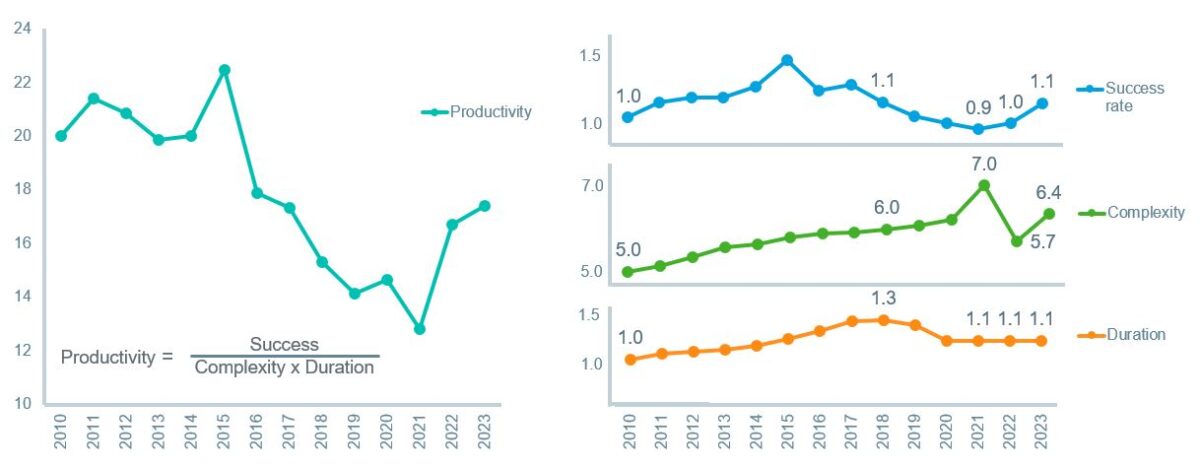
Source: Global Trends in R&D Overview Report by the IQVIA Institute for Human Data Science.
Key drivers of this turnaround include flexible trial models like adaptive designs, which allow for mid-study adjustments to optimize resource allocation and decision-making. Biomarker-driven stratification also enables trials to target specific subgroups, improving success rates while reducing costs.
The COVID-19 pandemic accelerated these trends, pushing the adoption of hybrid and virtual trial models. Decentralized trials, where participants engage via digital platforms, have grown significantly and accounted for 18% of trials from 2021 to 2023, reducing logistical burdens and enhancing accessibility. Oncology has been a particularly fertile ground for these innovations, with 29% of trials in the field adopting novel designs between 2021 and 2023.
Novel trial designs have averaged 18% of trials since 2020, led by oncology with over 29% novel designs
Novel trial design starts by year and therapy area, 2014-2023
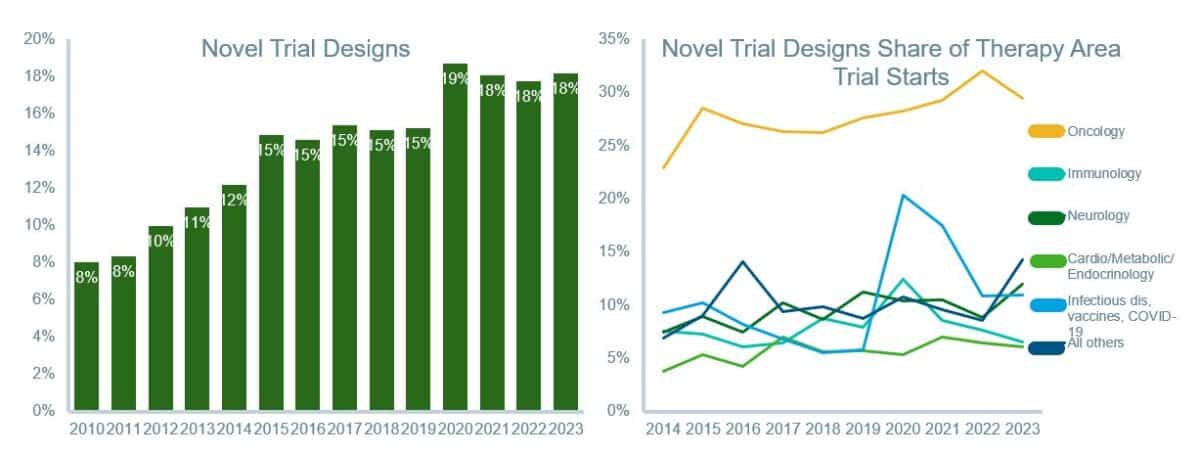
Source: Global Trends in R&D Overview Report by the IQVIA Institute for Human Data Science.
N-of-1 trials, where multiple crossover trials are conducted on a single patient, have gained traction as a promising approach to precision medicine. These individualized studies use advanced analytics and wearable technology to assess drug efficacy, reducing costs and providing highly relevant data. Regulatory agencies and pharmaceutical companies have also taken steps to improve efficiency, streamlining approval processes and optimizing trial site selection.
As the clinical trial industry evolves, the focus on adaptability and precision is transforming research into a faster, more patient-centered process. Israel’s startups, with their innovative approaches, are well positioned to shape this transformation on a global scale.
Israel’s Ecosystem: The Engine of Innovation
Israel has become a global leader in healthcare technologies, thanks to its unique intersection of technological innovation, diverse population, and advanced healthcare infrastructure.
At the heart of this leadership is Israel’s unparalleled “genetic mosaic” – a diverse population that provides an exceptional foundation for personalized healthcare. This diversity, combined with decades of digitized health records and advanced data systems, creates a research ecosystem capable of generating insights that few countries can replicate. As one of the top 5 countries in the number of clinical trials per capita—almost twice as high as the U.S.—Israel is not just keeping pace; it’s setting the standard.
Israel’s healthcare infrastructure further bolsters its leadership. Hospitals and research centers operate as interconnected hubs, supported by advanced electronic health records. This integration enables seamless collaboration, streamlined trial processes, and faster, more accurate research outcomes. Close partnerships between these institutions and Israeli startups facilitate innovation at every stage of the clinical trial lifecycle, from recruitment to regulatory approvals.
Fueling this ecosystem is an abundance of talent. Israel is home to a high concentration of engineers, data scientists, and AI experts who bring an interdisciplinary approach to healthcare innovation. These professionals, supported by experienced investors and government backing, power a thriving startup landscape. Israeli companies are transforming the clinical trial industry with AI-driven patient stratification, real-time safety monitoring, and decentralized trial solutions.
This strategic combination of talent, technology, and infrastructure positions Israel as a hub for clinical trial operations.
Beyond the advantages of advanced healthcare infrastructure, diverse patient data, and a rich talent pool, Israeli startups benefit from direct access to clinical trial sites that operate at or above the global gold standard in quality, accuracy, and scale. This combination provides an exceptional foundation for innovation, giving Israeli companies a significant advantage on the global stage, keeping it at the forefront of global healthcare innovation.
The Israeli Clinical Trial Operations Tech Landscape
Israel’s clinical trial ecosystem thrives on a combination of technological innovation, talent, and infrastructure. The local landscape in Israel has shown steady growth, with an increasing number of active companies dedicated to advancing trial processes and outcomes. Over the past decade, the sector has expanded significantly, from just 16 companies in 2014 to over 50 in recent years, highlighting Israel’s rising prominence in clinical research innovation.
The clinical trial operations tech ecosystem consists of a mix of companies in various stages, with 50% in the early stage, 29% in the early growth stage, and the remaining 20% in mature stages. 61% of these companies are small to medium-sized, with 11 to 50 employees, while 23% have over 50 employees. This heterogeneity contributes to new ideas while established players drive sustained growth.
Funding trends in recent years in clinical trial operation startups, mostly align with the general tech ecosystem trends, including the sharp peak in 2021 and a drop in 2022 and 2023. However, in 2024 there is a notable recovery in this domain with funding amounts higher than in 2020 and more than double the amount in 2023. This is mainly driven by a much higher funding amount per round.
Top Funded Clinical Trial Operations Tech Startups
Immunai is the top funded startup in this domain with $295M raised. This startup leverages AI to decode the immune system, which helps optimize clinical trials and preclinical asset evaluation. Other top funded startups are CytoReason who raised $132M, and C2i Genomics who raised $113M before being acquired by Veracyte earlier this year. C2i Genomics empowers researchers and physicians, and accelerates drug development by using clinical databases, as well as sophisticated signal processing and artificial intelligence.
Israeli Clinical Trial Operations: Five Key Segments
Israel is a hub for cutting-edge solutions across various clinical trial domains, as illustrated in the following segments:

ACCESS A FULL LIST OF CLINICAL TRIAL TECHNOLOGY COMPANIES ON FINDER
1. Novel Trial Designs and Patient Stratification
Traditional trial designs often lack the flexibility needed to adapt to emerging insights, leading to inefficiencies and delays. Israeli innovations in adaptive, basket, and umbrella trials enable more dynamic study structures, allowing for adjustments mid-trial and focusing on patient subgroups that are most likely to benefit. These approaches streamline the trial process and align with the global shift toward precision medicine.
- PhaseV: Helps improve the design, execution, and overall success rate of clinical trials by detecting hidden signals in clinical data and optimizing patient eligibility.
- QuantHealth: Utilizes a Large Healthcare Model (LHM) to learn patient-drug interactions and develop high-resolution outcome models. Their Clinical Trial Simulator enhances clinical trials simulations.
- CytoReason: Uses AI-driven computational disease models to refine patient selection, ensuring trials target the most responsive subgroups.
2. Patient Recruitment and Retention
Recruiting and retaining participants is one of the most significant challenges in clinical trials, often causing delays and escalating costs. Israeli startups are tackling this issue with advanced analytics, behavioral science, and AI-powered platforms that match participants to trials efficiently and maintain engagement through personalized support.
- Leal Health: Leverages AI to match patients with suitable clinical trials based on their medical profiles, significantly reducing recruitment timelines.
- Sweetch: Applies behavioral science to predict and influence patient engagement, ensuring consistent participation throughout trials.
- Belong: Provides a mobile platform that delivers trial information and personalized support, fostering a strong connection between participants and researchers.
3. Safety and Pharmacovigilance
Ensuring participant safety is critical to trial success and regulatory compliance. Israeli technologies enhance pharmacovigilance with real-time monitoring and advanced analytics that detect adverse events quickly and reliably.
- Medisafe: Captures and monitors medication data to optimize safety profiles during trials.
- MDI Health: Uses AI to identify high-risk participants and prevent adverse drug events, improving overall trial safety.
- Clinicode: Automates the detection and reporting of adverse events, streamlining compliance with regulatory standards.
4. Data Management and Analytics
Effective data management is essential for modern trials, particularly as decentralized and hybrid models become more prevalent. Israeli companies are transforming this space with cloud-based, secure, and scalable solutions that facilitate real-time data sharing and analysis.
- Briya Health: Integrates diverse datasets to provide actionable insights that enhance trial efficiency and outcomes.
- YonaLink: Bridges the gap between electronic health records and trial databases, enabling seamless data integration for decentralized studies.
- Rhino Health: Employs federated learning to ensure secure data analysis across multiple sites while maintaining privacy compliance.
5. Logistics and Regulatory Affairs
Operational complexities in clinical trials, including logistics and regulatory submissions, can slow down progress and inflate costs. Israeli startups offer solutions that simplify these processes, ensuring trials are conducted efficiently and on schedule.
- Beaconcure: Automates data validation to expedite regulatory approvals, reducing the burden on research teams.
- Next-Step Tech: Optimizes clinical trial logistics, ensuring smooth supply chain operations and timely delivery of trial materials.
Key segments Funding
Trial Designs is the leading segment with 19 startups and $824M raised in funding and accounting for 56% of the total funding that was invested in Clinical Trial Operations tech companies. Other sizable segments are Patient Relationship and Trial Operations, which together consist of 22 startups and a total $440M raised.
Israel’s Strategic Advantage
Israel stands as a global leader in clinical trial innovation, combining advanced technology, a diverse talent pool, and unmatched healthcare infrastructure. Its startups address critical challenges in trial design, recruitment, safety, data management, and operations, offering scalable solutions that meet global demands. As the industry evolves toward more patient-centered, adaptable approaches, Israel’s contributions remain pivotal in shaping the future of clinical research.
We wish to thank the following individuals for their contribution: Yoav Fischer, Lena Rogovin, Matan Eblagon, and Eran Igelnik
